
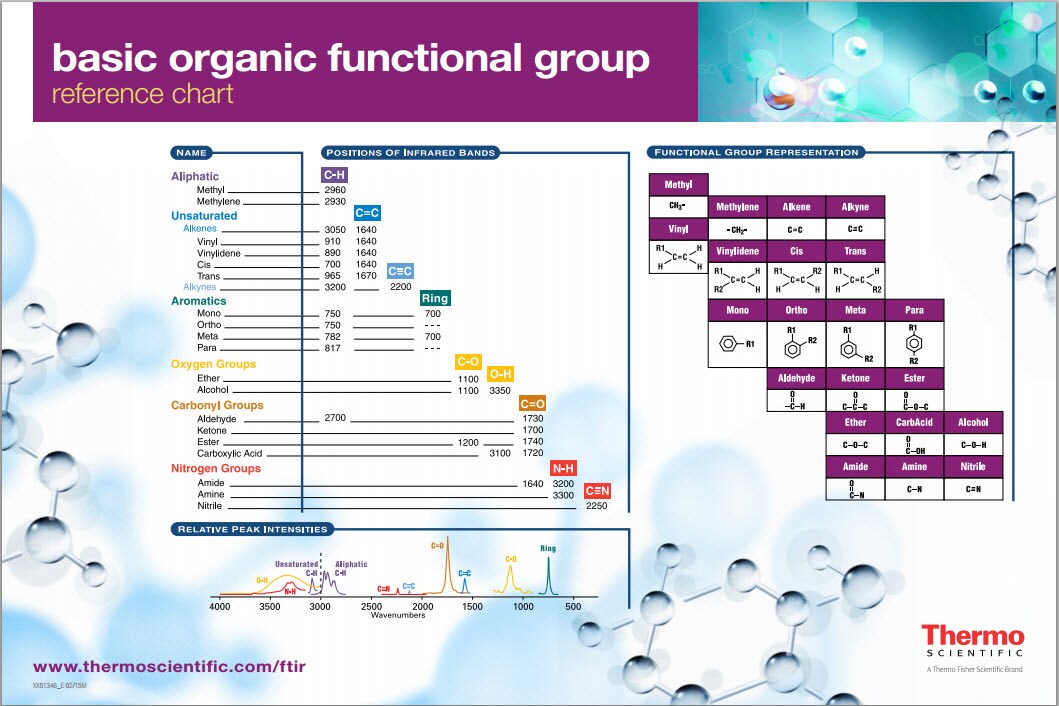
The region of the infrared spectrum from 1200 to 700 cm-1 is called the fingerprint region. Group frequency and fingerprint regions of the mid-infrared spectrum. When analyzing an IR spectrum, it is helpful to overlay the diagram below onto the spectrum with our mind to help recognize functional groups. Common Group Frequencies Summary.
Alcohols have IR absorptions associated with both the O-H and the C-O stretching vibrations. Since most organic compounds have C-H bonds, a useful rule is that absorption in the 2850 to 3000 cm -1 is due to sp 3 C-H stretching whereas, absorption above 3000 cm -1 is from sp 2 C-H stretching or sp C-H stretching if it is near 3300 cm -1.Functional Groups Containing the C-O Bond. Alkenes, arenes, alcohols, amines & carbonyl compounds) may be viewed by clicking on the functional class name. More detailed descriptions for certain groups (e.g. Following the color scheme of the chart, stretching absorptions are listed in the blue-shaded section and bending absorptions in the green shaded part. The following table provides a collection of such data for the most common functional groups.
C–H bend or scissoring from 1470-1450 cm -1 Note the very broad, strong band of the OH stretch.FTIR spectroscopy is a form of the vibrational spectroscopic technique commonly used to determine the functional groups present in the composites 72.Hydrocarbons compounds contain only C-H and C-C bonds, but there is plenty of information to be obtained from the infrared spectra arising from C-H stretching and C-H bending.In alkanes, which have very few bands, each band in the spectrum can be assigned: Shows the spectrum of ethanol.
Shows the IR spectrum of 1-octene. Infrared Spectrum of OctaneIn alkenes compounds, each band in the spectrum can be assigned:Figure 4. Note that the change in dipole moment with respect to distance for the C-H stretching is greater than that for others shown, which is why the C-H stretch band is the more intense. Since most organic compounds have these features, these C-H vibrations are usually not noted when interpreting a routine IR spectrum. Shows the IR spectrum of octane.
C–C stretch (in-ring) from 1600-1585 cm -1 Infrared Spectrum of 1-HexyneIn aromatic compounds, each band in the spectrum can be assigned: –C?C–H: C–H stretch from 3330-3270 cm -1The spectrum of 1-hexyne, a terminal alkyne, is shown below. Infrared Spectrum of 1-OcteneIn alkynes, each band in the spectrum can be assigned:
Shows the spectrum of ethanol. O–H stretch, hydrogen bonded 3500-3200 cm -1Figure 7. Infrared Spectrum of TolueneFunctional Groups Containing the C-O BondAlcohols have IR absorptions associated with both the O-H and the C-O stretching vibrations. Shows the spectrum of toluene. Only alkenes and aromatics show a C–H stretch slightly higher than 3000 cm -1.Figure 6. This is a very useful tool for interpreting IR spectra.
Shows the spectrum of 2-butanone. C=O stretch - aliphatic ketones 1715 cm -1- ?, ?-unsaturated ketones 1685-1666 cm -1Figure 8. Infrared Spectrum of EthanolThe carbonyl stretching vibration band C=O of saturated aliphatic ketones appears:
Infrared Spectrum of Ethyl benzoateThe carbonyl stretch C=O of a carboxylic acid appears as an intense band from 1760-1690 cm -1. Shows the spectrum of ethyl benzoate. Infrared Spectrum of ButyraldehydeThe carbonyl stretch C=O of esters appears:Figure 10. Shows the spectrum of butyraldehyde. alpha, beta-unsaturated aldehydes 1710-1685 cm -1Figure 9. Infrared Spectrum of 2-ButanoneIf a compound is suspected to be an aldehyde, a peak always appears around 2720 cm -1 which often appears as a shoulder-type peak just to the right of the alkyl C–H stretches.
How can you distinguish the following pairs of compounds through IR analysis?A) CH 3OH (Methanol) and CH 3CH 2OCH 2CH 3 (Diethylether)3. What functional groups give the following signals in an IR spectrum?2. Infrared Spectrum of Hexanoic acid1. Shows the spectrum of hexanoic acid. O–H bend from 1440-1395 and 950-910 cm -1Figure 11.


 0 kommentar(er)
0 kommentar(er)
